Iantrek Hits 2,000 Procedures for New Bio-Interventional Technology - AlloFlo™ Uveo
CEO Adam Szaronos provides a technology update at the Eyecelerator Conference
/EIN News/ -- WHITE PLAINS, N.Y., May 06, 2025 (GLOBE NEWSWIRE) -- Iantrek, Inc., a leader in bio-interventional ophthalmic surgery (BIOS), today announced it has surpassed 2,000 AlloFlo™ Uveo procedures in the U.S., a key milestone ahead of its full commercial launch planned for this fall during the American Academy of Ophthalmology 2025 (AAO). Recent momentum has been further fueled by the publication of 2-year safety and efficacy data and the American Academy of Ophthalmology's new reimbursement endorsement for cyclodialysis with scleral reinforcement—solidifying the clinical and economic foundation for widespread adoption.
“I’m incredibly excited to see the momentum building around AlloFlo Uveo,” said Adam Szaronos, CEO of Iantrek. “This innovation is a much overdue solution to a material science challenge — using natural homologous biologic tissue to reinforce a critical outflow pathway largely untapped in the interventional glaucoma treatment paradigm. Surgeons are embracing this as a solution that may help millions of glaucoma patients who are experiencing waning efficacy following canal-based MIGS.”
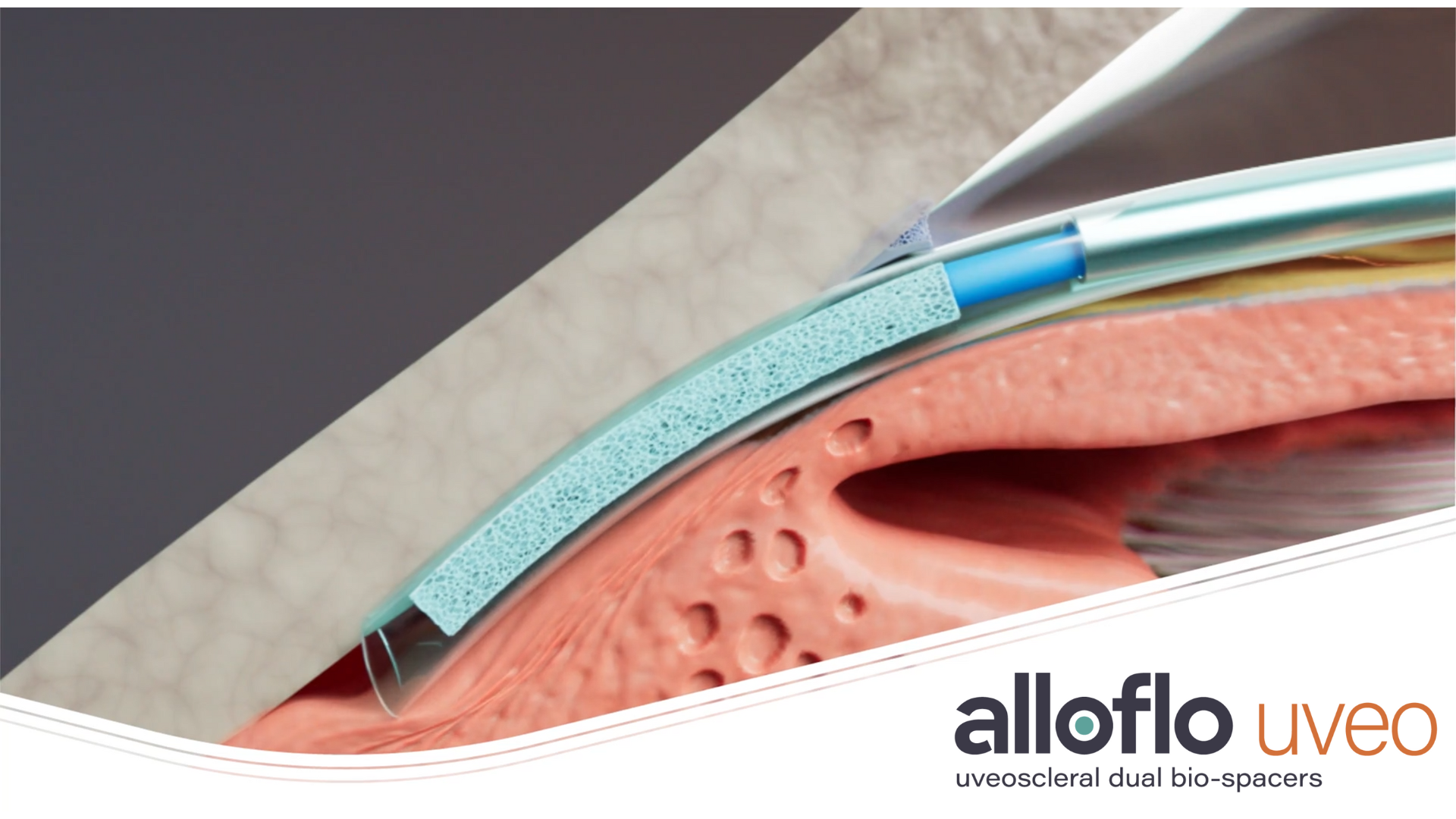
AlloFlo Uveo is part of Iantrek’s proprietary Allo platform and is designed to enhance the uveoscleral outflow pathway through scleral reinforcement following controlled cyclodialysis. As the first homologous, bio-conforming solution engineered for uveoscleral outflow enhancement, AlloFlo Uveo offers a hardware-free, naturally-derived bio-tissue solution for patients suffering from glaucoma.
“Surgeons have long awaited a way to surgically enhance uveoscleral outflow,” said Dr. Peter Chang, of Baylor College of Medicine, “Today, that need is more pressing than ever. With MIGS having been offered to patients for over a decade, there are now more than 2.5 million eyes in the U.S. experiencing waning efficacy that may benefit from the addition of AlloFlo Uveo to our surgical armamentarium.”
At this year’s AAO, Iantrek will officially launch, celebrate early clinical adopters, and offer a preview of upcoming pipeline innovations designed to build on the Allo platform’s unique potential. Reach out to media contact to learn more or schedule a meeting during AAO 2025.
About Iantrek, Inc.
Iantrek is a venture-funded medical technology company founded by ophthalmic innovator Sean Ianchulev, MD, MPH. Iantrek is pioneering a novel platform of bio-interventional and micro-interventional products for ophthalmic surgery. The company’s portfolio includes CycloPen™, AlloFlo™, CanaloFlo™ and C.Rex™, representing next-generation, hardware-free solutions for the interventional management of glaucoma. For more information, visit iantrekmed.com
Media contact:
Mike Haydin
mikehaydin@iantrekmed.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/fd943b5b-150f-4ff9-8264-a8ad2be5a9a8

Distribution channels: Media, Advertising & PR, Technology ...
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release